Abstract
Background
Acute graft-versus-host disease (aGVHD) remains a major limitation of allogeneic hematopoietic cell transplantation (allo-HCT) despite prophylactic immunosuppression. Not all patients who develop aGVHD respond to currently available treatment and thus there is a pressing need for novel prophylactic and therapeutic strategies.
Interleukin-2-Inducible T-Cell Kinase (ITK) is a Tec-family, non-receptor tyrosine kinase expressed in T-cells, homologous with Bruton's tyrosine kinase (BTK) in B cells and is involved in proximal T-cell receptor (TCR) signaling, activating Phospholipase Cγ1 (PLCγ1) and initiating a signaling cascade that includes the NFAT, NF-κB, and MAPK pathways tuning T-cell activation, proliferation, and differentiation. Targeting ITK (ITK-/- in T cell graft and short-term exposure of donor graft to ITK inhibitor) has been shown to be associated with less GVHD in preclinical studies (Mammadli et al 2020, Kondo et al 2021).
CPI-818 is a highly selective irreversible ITK inhibitor that covalently binds to cysteine 442 of ITK and abolishes kinase activity. In vitro assays demonstrate that CPI-818 inhibits phosphorylation of the ITK substrate PLC γ1 (Y783) and downstream signaling molecules ERK and S6, and blocks IL-2 secretion. CPI-818 reduced clinical and histological disease severity in a Th1-driven T cell adoptive transfer model of colitis (not published). CPI-818 is currently being evaluated in a Phase 1/1b study in patients with relapsed/refractory T-cell lymphoma (NCT03952078), and thus far appears well tolerated.
Methods and Results
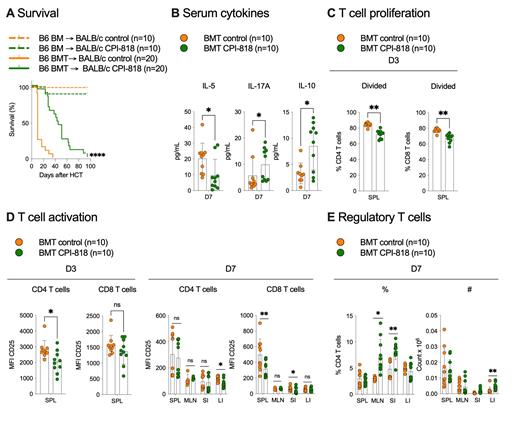
We used 2 well established preclinical aGVHD models (MHC-disparate B6 → BALB/c and minor MHC-mismatched B6 → 129) to explore the effects of ITK inhibition by CPI-818. Recipient mice were treated with CPI-818-formulated (300 mg/kg/day) or control diet from day -7 to day 90 relative to allo-HCT. We found significantly improved survival (MHC-disparate: p<0.0001, n=20/group, 2 independent experiments, Figure A; minor mismatch: p=0.019, n=20/group, 2 independent experiments), in mice treated with CPI-818 compared with control. In the MHC-disparate model we observed reduced serum concentrations of IL-5 and IL-17a on day 7 after allo-HCT (IL-5: 6.9 vs 22.1 pg/mL, p=0.048; IL-17a: 2.7 vs 9.2 pg/mL, p=0.037, 10 mice/group, Figure B). When we analyzed T cells in the spleen, 3 days post transplant, we saw reduced proliferation of both CD4 and 8 T cells as measured by CFSE dilution (CD4: 73.0 vs 85.1%, p=0.002, CD8: 53.5 vs 65.1%, p=0.002, n=10/group, Figure C), accompanied by a reduction in CD4 T cell expression of CD25 (MFI CD25, 2034.5 vs 2693.5, p=0.037, 10 mice/group, Figure D). By day 7, we observed reduced T cell activation in the small and large intestine (p<0.02, n=10/group, Figure D). Conversely, CPI-818 treated mice had increased serum concentrations of anti-inflammatory IL-10 (Day 7, 10.1 vs 3.1 pg/mL, p=0.012, n=10/group, Figure B) and increased proportions of FoxP3+ T reg in mesenteric lymph nodes (6.2 vs 3.1%, p=0.002, n=10/group, Figure E) and both small and large intestines (small intestine: p=0.01, n=10/group; large intestine: p=0.004, Figure E) on day 7 after allo-HCT. In vitro, 1uM CPI-818 reduced proliferation of T cells exposed to LPS-activated allogeneic DCs compared with vehicle control (CD4: 73.0 vs 81.4%, p=0.008; CD8: 90.1 vs 93.9%, p=0.012; data from 3 independent experiments). This was also seen when T cells were stimulated with CD3/CD28 beads (CD4: 66.2 vs 81.3%, p=0.03; CD8: 67.3 vs 90.6, p=0.03; data from 2 independent experiments) and was not caused by reduced viability of T cells due to drug exposure.
Conclusion
These data in two clinically relevant GVHD models demonstrate that ITK inhibition has potential as a novel targeted approach to prevent aGVHD through a) the suppression of T cell activation and proliferation, b) decreased concentrations of pro-inflammatory cytokines and increased concentration of anti-inflammatory cytokines. CPI-818 is the most potent and selective ITK inhibitor reported to date and these data highlight its promise as a novel agent for the prevention of aGVHD.
Smith: Janssen: Consultancy, Honoraria. Perales: Celgene: Honoraria; Kite/Gilead: Honoraria, Other; MorphoSys: Honoraria; Merck: Honoraria; Incyte: Honoraria, Other; Cidara: Honoraria; Bristol-Myers Squibb: Honoraria; Omeros: Honoraria; Novartis: Honoraria, Other; Medigene: Honoraria; Karyopharm: Honoraria; Nektar Therapeutics: Honoraria, Other; Equilium: Honoraria; Takeda: Honoraria; Sellas Life Sciences: Honoraria; NexImmune: Honoraria; Miltenyi Biotec: Honoraria, Other; Servier: Honoraria. Buggy: Corvus Pharmaceuticals Inc: Consultancy, Current holder of individual stocks in a privately-held company. Janc: Corvus Pharmaceuticals Inc: Current Employment, Current holder of individual stocks in a privately-held company. van den Brink: Forty-Seven, Inc.: Honoraria; WindMILTherapeutics: Honoraria; Kite Pharmaceuticals: Other; Da Volterra: Other: has consulted, received honorarium from or participated in advisory boards; Notch Therapeutics: Honoraria; Pluto Therapeutics: Current holder of stock options in a privately-held company, Other: has consulted, received honorarium from or participated in advisory boards ; GlaskoSmithKline: Other: has consulted, received honorarium from or participated in advisory boards; Synthekine (Spouse): Other: has consulted, received honorarium from or participated in advisory boards; Priothera: Research Funding; Wolters Kluwer: Patents & Royalties; Pharmacyclics: Other; Rheos: Honoraria; Nektar Therapeutics: Honoraria; Ceramedix: Other: has consulted, received honorarium from or participated in advisory boards ; Novartis (Spouse): Other: has consulted, received honorarium from or participated in advisory boards; Lygenesis: Other: has consulted, received honorarium from or participated in advisory boards ; Frazier Healthcare Partners: Honoraria; DKMS (nonprofit): Other; Juno Therapeutics: Other; MagentaTherapeutics: Honoraria; Merck & Co, Inc: Honoraria; Amgen: Honoraria; Therakos: Honoraria; Jazz Pharmaceuticals: Honoraria; Seres: Other: Honorarium, Intellectual Property Rights, Research Fundingand Stock Options.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal